Multiple Choice
How much copper, in g, can be plated from a solution of 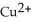 if 3.14 A is applied for 45 minutes?
if 3.14 A is applied for 45 minutes?
A) 2.79 g
B) 1.85 g
C) 11.2 g
D) 0.283 g
E) 7.64 g
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q18: Use the standard half-cell potentials listed below
Q81: Calculate the cell potential for the following
Q82: What is the reducing agent in the
Q84: Calculate the cell potential for the following
Q87: Predict the species that will be reduced
Q88: Determine the cell notation for the redox
Q89: Consider the galvanic cell, <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB7901/.jpg" alt="Consider
Q90: Which of the following is the weakest
Q94: Why are iron nails coated with zinc?
Q113: How many grams of chromium metal are