Multiple Choice
A voltaic cell is constructed with two silver-silver chloride electrodes, where the half-reaction is AgCl(s) + 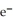 → Ag(s) +
→ Ag(s) + 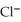 (aq) E° = +0.222 V
(aq) E° = +0.222 V
The concentrations of chloride ion in the two compartments are 0.0557 mol L-1 and 5.31 mol L-1, respectively. The cell emf is ________ V.
A) 0.00557
B) 0.531
C) 53.1
D) 0.234
E) 0.117
Correct Answer:

Verified
Correct Answer:
Verified
Q1: Which of the following metals will dissolve
Q1: Match the following.<br>-Q > K<br>A)E<sub>cell</sub> = E°<sub>cell</sub><br>B)E<sub>cell</sub>
Q2: Which of the following is the strongest
Q3: Calculate the cell potential for the following
Q6: What is the shorthand notation that represents
Q10: Predict the species that will be reduced
Q11: Match the following.<br>-Δ<sub>r</sub>G° < 0<br>A)E<sub>cell</sub> = E°<sub>cell</sub><br>B)E<sub>cell
Q16: Using the following standard reduction potentials,<br>Fe<sup>3+</sup>(aq)+ e<sup>-</sup>
Q21: What is electrolysis?
Q115: Match the following.<br>-Q = 1<br>A)E<sub>cell</sub> = E°<sub>cell</sub><br>B)E<sub>cell</sub>