Multiple Choice
A voltaic cell is constructed with two 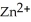 -Zn electrodes, where the half-reaction is
-Zn electrodes, where the half-reaction is 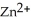 +
+ 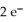 → Zn (s) E° = -0.763 V The concentrations of zinc ion in the two compartments are 1.17 ×
→ Zn (s) E° = -0.763 V The concentrations of zinc ion in the two compartments are 1.17 × 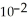 mol L-1 and 8.70 ×
mol L-1 and 8.70 × 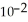 mol L-1, respectively. The cell emf is ________ V.
mol L-1, respectively. The cell emf is ________ V.
A) 0.170
B) -0.678
C) -0.0850
D) 515
E) 0.0850
Correct Answer:

Verified
Correct Answer:
Verified
Q6: How long must a constant current of
Q35: Nickel can be plated from aqueous solution
Q73: What is the balanced equation for the
Q75: Consider the following standard reduction potentials:<br>Ni<sup>2+</sup>(aq)+ 2
Q90: Which of the following is the weakest
Q91: Calculate the cell potential for the following
Q93: Calculate the cell potential for the following
Q99: Calculate the cell potential for the following
Q100: Calculate the cell potential for the following
Q151: What is the reaction at the cathode