Multiple Choice
Use the following thermodynamic values to calculate Δr 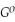 .
.
Δr
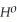 = +95 kJ
= +95 kJ 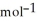 , Δr
, Δr
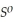 = -157 J
= -157 J 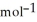 , T = 398 K
, T = 398 K
A) -48 kJ 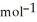
B) -68 kJ 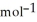
C) +39 kJ 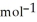
D) +157 kJ 
E) +142 kJ 
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q13: Identify the compound with the lowest standard
Q14: Use the following thermodynamic values to calculate
Q15: What is the name of the reaction
Q16: Determine Δ<sub>r</sub>G° at 298 K using the
Q17: Calculate Δ<sub>r</sub>S° for the following reaction. The
Q19: Consider a reaction that has a positive
Q20: Calculate Δ<sub>r</sub>G° at 298 K using the
Q22: For a given reaction, Δ<sub>r</sub>H = -19.5
Q23: For the following example, what is true
Q89: What is "free" energy? Give a fictitious