Multiple Choice
The value of ΔfG° at 161.0 °C for the formation of phosphorus trichloride from its constituent elements, 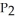 (g) +
(g) + 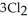 (g) →
(g) → 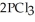 (g) is ________ kJ mol-1. At 25.0 °C for this reaction, ΔfH° is -720.5 kJ mol-1 and
(g) is ________ kJ mol-1. At 25.0 °C for this reaction, ΔfH° is -720.5 kJ mol-1 and 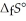 is
is 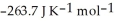 .
.
A) -678.0
B) -834.9
C) 1.14 × 105
D) 4.17 × 104
E) -606.1
Correct Answer:

Verified
Correct Answer:
Verified
Q37: Why can't we say that a spontaneous
Q48: Determine the equilibrium constant for the following
Q50: Which one of the following has the
Q51: Calculate Δ<sub>r</sub>G for the evaporation of methanol
Q52: Which of the following is TRUE if
Q54: Which of the following is TRUE if
Q55: Calculate Δ<sub>r</sub>G at 298 K under the
Q56: Determine the equilibrium constant for the following
Q57: Below what temperature does the following reaction
Q58: What is the sign of ΔS<sub>univ</sub> for