Multiple Choice
The value of ΔfG° at 400.0 °C for the formation of calcium chloride from its constituent elements, Ca(s) + 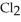 (g) →
(g) → 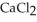 (s)
(s)
Is ________ kJ mol-1. At 25.0 °C for this reaction, ΔfH° is -795.8 kJ mol-1, ΔfG° is -748.1 kJ mol-1, and 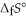 is
is 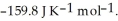
A) -731.9
B) -903.3
C) 1.07 × 105
D) 6.31 × 104
E) -688.3
Correct Answer:

Verified
Correct Answer:
Verified
Q53: Why is heating your home with gas
Q83: Define the third law of thermodynamics.
Q93: Calculate Δ<sub>r</sub>G for the evaporation of methanol
Q94: Determine the Q of a reaction at
Q95: Identify the compound with the highest standard
Q96: Which of the following processes have a
Q99: Calculate Δ<sub>r</sub>G° at 298 K using the
Q101: Use the free energies of formation given
Q102: Consider a reaction that has a positive
Q132: Match the following.<br>-Q = 1<br>A)standard state<br>B)equilibrium<br>C)Δ<sub>r</sub>G >