Multiple Choice
In the Haber process, ammonia is synthesized from nitrogen and hydrogen: 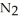 (g) +
(g) + 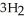 (g) →
(g) → 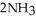 (g) ΔrG° at 298 K for this reaction is -33.3 kJ mol-1. The value of ΔrG at 298 K for a reaction mixture that consists of 1.7 atm
(g) ΔrG° at 298 K for this reaction is -33.3 kJ mol-1. The value of ΔrG at 298 K for a reaction mixture that consists of 1.7 atm 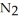 , 1.1 atm
, 1.1 atm 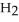 , and 0.55 atm
, and 0.55 atm 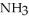 is ________ kJ mol-1.
is ________ kJ mol-1.
A) -5.02 × 103
B) -4.5
C) -72.2
D) -35.3
E) -38.3
Correct Answer:

Verified
Correct Answer:
Verified
Q74: Estimate Δ<sub>r</sub>G° for the following reaction at
Q75: What is the change in Gibbs energy
Q76: Given the following equation, N<sub>2</sub>O(g) + NO<sub>2</sub>(g)
Q77: Calculate Δ<sub>r</sub>G° at 298 K using the
Q80: Which of the following processes shows a
Q81: Above what temperature does the following reaction
Q82: Which one of the following has the
Q83: For the following example, what is true
Q84: Calculate Δ<sub>r</sub>G° at 298 K using the
Q127: Place the following in order of increasing