Multiple Choice
Phosphorus and chlorine gases combine to produce phosphorus trichloride: 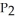 (g) +
(g) + 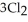 (g) →
(g) → 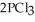 (g) ΔrG° at 298 K for this reaction is -642.9 kJ mol-1. The value of ΔrG at 298 K for a reaction mixture that consists of
(g) ΔrG° at 298 K for this reaction is -642.9 kJ mol-1. The value of ΔrG at 298 K for a reaction mixture that consists of 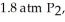
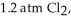 and
and 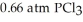 is ________ kJ mol-1.
is ________ kJ mol-1.
A) -5.51 × 103
B) -90.0
C) -71.1
D) -4.87 × 103
E) -647.8
Correct Answer:

Verified
Correct Answer:
Verified
Q104: Define entropy.
Q124: Determine the equilibrium constant for the following
Q125: For the following example, what is true
Q126: Consider a reaction that has a positive
Q127: What is the name of the reaction
Q128: Determine Δ<sub>r</sub>G° at 298 K using the
Q131: Estimate Δ<sub>r</sub>G° for the following reaction at
Q132: Match the following.<br>-Q = 1<br>A)standard state<br>B)equilibrium<br>C)Δ<sub>r</sub>G >
Q133: Calculate the equilibrium constant for the following
Q134: Determine the temperature of a reaction if