Multiple Choice
An acid solution is 0.100 mol L-1 in HCl and 0.200 mol L-1 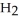
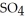 . What volume of a 0.150 mol L-1 KOH solution would completely neutralize 500.0 mL of this solution?
. What volume of a 0.150 mol L-1 KOH solution would completely neutralize 500.0 mL of this solution?
A) 0.700 L
B) 1.98 L
C) 1.67 L
D) 1.02 L
E) 0.250 L
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q124: Identify the salts that are in hard
Q125: A buffer solution is 0.100 mol L<sup>-1</sup>
Q126: Calculate the pH of a buffer that
Q127: If the pK<sub>a </sub>of HCOOH is 3.74
Q128: Sodium hypochlorite, NaOCl, is the active ingredient
Q130: Determine the molar solubility of CaSO<sub>4</sub> in
Q131: A 100.0 mL sample of 0.18 mol
Q132: A sample contains Ba<sub>3</sub>(PO<sub>4</sub>)<sub>2</sub>,<sub> </sub>CdS, AgCl, NH<sub>4</sub>Cl,
Q133: A 100.0 mL sample of 0.10 mol
Q134: Calculate the pH of a buffer that