Multiple Choice
Calculate the percent ionization of nitrous acid in a solution that is 0.210 mol L-1 in nitrous acid 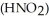 and 0.290 mol L-1 in potassium nitrite (
and 0.290 mol L-1 in potassium nitrite ( 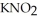 ) . The acid dissociation constant of nitrous acid is
) . The acid dissociation constant of nitrous acid is 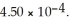
A) 58.0
B) 0.154
C) 16.6
D) 2.74 × 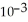
E) 1.55
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q143: What is the pH of a solution
Q144: Identify a good buffer.<br>A) a solution containing
Q145: Calculate the mole ratio of HCOONa to
Q146: The molar solubility of CuI is 2.26
Q147: How many millilitres of 0.0890 mol L<sup>-1</sup>
Q149: Describe the solubility of Al(OH)<sub>3</sub> with respect
Q150: A solution is prepared by dissolving 0.23
Q151: A 100.0 mL sample of 0.10 mol
Q152: Calculate the pH of a buffer that
Q153: A 100.0 mL sample of 0.180 mol