Multiple Choice
Calculate the percent ionization of nitrous acid in a solution that is 0.249 mol L-1 in nitrous acid. The acid dissociation constant of nitrous acid is 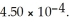
A) 5.53 %
B) 0.0425 %
C) 1.12 × 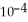 %
%
D) 4.25 %
E) 0.0450 %
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q1: What is the pH of a buffer
Q2: What is the molar solubility of AgCl
Q2: What is the pH of a buffer
Q3: Determine the molar solubility of AgBr in
Q5: What is the pH of the resulting
Q6: What is the maximum ratio of conjugate
Q7: A solution containing CaCl<sub>2</sub> is mixed with
Q8: What is the pH of a solution
Q10: Calculate the K<sub>sp</sub> for silver carbonate if
Q11: When titrating a strong monoprotic acid with