Multiple Choice
What is the hydronium ion concentration in a solution prepared by mixing 50.00 mL of 0.10 mol L-1 HCN with 50.00 mL of 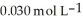 NaCN? Assume that the volumes of the solutions are additive and that Ka =
NaCN? Assume that the volumes of the solutions are additive and that Ka = 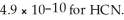
A) 1.5 × 10-10 mol L-1
B) 4.9 × 10-10 mol L-1
C) 1.6 × 10-9 mol L-1
D) 7.0 × 10-6 mol L-1
Correct Answer:

Verified
Correct Answer:
Verified
Q117: Give the name of the compound that
Q130: Determine the molar solubility of CaSO<sub>4</sub> in
Q131: A 100.0 mL sample of 0.18 mol
Q132: A sample contains Ba<sub>3</sub>(PO<sub>4</sub>)<sub>2</sub>,<sub> </sub>CdS, AgCl, NH<sub>4</sub>Cl,
Q133: A 100.0 mL sample of 0.10 mol
Q134: Calculate the pH of a buffer that
Q136: Identify the indicator that can be used
Q138: A sample contains Ba<sub>3</sub>(PO<sub>4</sub>)<sub>2</sub>,<sub> </sub>CdS, AgCl, NH<sub>4</sub>Cl,
Q139: Which of the following acids (listed with
Q140: A 100.0 mL sample of 0.18 mol