Multiple Choice
How many millilitres of 0.120 mol L-1 NaOH are required to titrate 50.0 mL of 0.0998 mol L-1 acetic acid to the equivalence point? Acetic acid is monoprotic. The 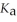 of acetic acid is 1.8 × 10-5.
of acetic acid is 1.8 × 10-5.
A) 41.6
B) 60.1
C) 50.0
D) 1.77
E) 2.82
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q117: A 100.0 mL sample of 0.20 mol
Q118: Identify the indicator that has two endpoints.<br>A)
Q119: Calculate the molar solubility of thallium chloride
Q120: Which of the following solutions is a
Q121: Calculate the mole ratio of CH<sub>3</sub>COONa to
Q123: A 100.0 mL sample of 0.10 mol
Q124: Identify the salts that are in hard
Q125: A buffer solution is 0.100 mol L<sup>-1</sup>
Q126: Calculate the pH of a buffer that
Q127: If the pK<sub>a </sub>of HCOOH is 3.74