Multiple Choice
A 25.0 mL sample of 0.150 mol L-1 formic acid is titrated with a 0.150 mol L-1 NaOH solution. What is the pH at the equivalence point? The 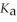 of formic acid is 1.8 × 10-4.
of formic acid is 1.8 × 10-4.
A) 11.38
B) 10.26
C) 5.69
D) 8.31
E) 7.00
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q21: If the pKa of HCOOH is 3.74
Q22: Determine the molar solubility of BaF<sub>2</sub> in
Q23: Which of the following is the correct
Q24: Which of the following acids (listed with
Q26: A 100.0 mL sample of 0.20 mol
Q27: If the pK<sub>a</sub> of HCOOH is 3.74
Q28: What is the pH of a solution
Q30: A sample contains Ba<sub>3</sub>(PO<sub>4</sub>)<sub>2</sub>,<sub> </sub>CdS, AgCl, NH<sub>4</sub>Cl,
Q157: When titrating a weak monoprotic acid with
Q173: Match the following.<br>-pH = 7<br>A)equivalence point of