Multiple Choice
A 25.0 mL sample of 0.150 mol L-1 butanoic acid is titrated with a 0.150 mol L-1 NaOH solution. What is the pH after 13.3 mL of base is added? The 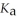 of butanoic acid is 1.5 × 10-5.
of butanoic acid is 1.5 × 10-5.
A) 1.34
B) 4.55
C) 3.08
D) 4.77
E) 4.88
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q16: Describe the information necessary (and why)to choose
Q36: What is the pH of the resulting
Q37: A solution containing AgNO<sub>3</sub> is mixed with
Q38: Calculate the mole ratio of C<sub>6</sub>H<sub>5</sub>COONa to
Q39: What is the approximate pH at the
Q40: What volume of 5.00 × 10<sup>-3</sup> mol
Q42: Define buffer capacity.<br>A) Buffer capacity is the
Q43: Identify the expression for the solubility product
Q45: Which of the following is the correct
Q46: For a strong acid titrated with a