Multiple Choice
Calculate the solubility (in g L-1) of silver carbonate in water at 25 °C if the Ksp for 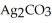 is 8.4 ×
is 8.4 × 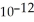 .
.
A) 8.0 × 10-4 g L-1
B) 3.5 × 10-2 g L-1
C) 4.4 × 10-2 g L-1
D) 5.6 × 10-2 g L-1
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q155: What is the pH of a solution
Q156: The titration of 80.0 mL of an
Q157: Determine the molar solubility of PbSO<sub>4</sub> in
Q158: Calculate the pH of a solution formed
Q159: Identify the most commonly used indicator among
Q161: The molar solubility of ZnS is 1.6
Q162: In which of the following solutions would
Q163: The titration of 25.0 mL of an
Q164: Which of the following is the correct
Q165: A 100.0 mL sample of 0.10 mol