Multiple Choice
What is the molar solubility of calcium hydroxide ( Ca(OH) 2) in water? The solubility-product constant for CaOH2 is 4.5 × 10-6 at 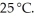
A) 1.5 × 10-6 mol L-1
B) 3.0 × 10-3 mol L-1
C) 1.1 × 10-3 mol L-1
D) 1.7 × 10-2 mol L-1
E) 1.0 × 10-2 mol L-1
Correct Answer:

Verified
Correct Answer:
Verified
Q51: Identify the compound that is acid-insoluble.<br>A) PbCl<sub>2</sub><br>B)
Q60: A solution contains 0.036 mol L<sup>-1</sup> Cu<sup>2+</sup>
Q61: You wish to prepare a buffer containing
Q62: Match the following.<br>-pH = pK<sub>a2</sub><br>A)equivalence point of
Q63: A 100.0 mL sample of 0.10 mol
Q64: Calculate the pH of a buffer that
Q67: Calculate the pH of a solution formed
Q69: A 1.0 L buffer solution is 0.050
Q70: Predict the number of equivalence point(s) in
Q136: Explain the common ion effect with respect