Multiple Choice
Solid potassium chromate (K2CrO4) is slowly added to a solution containing 0. 50 mol L-1 AgNO3 and 0. 50 mol L-1 Ba(NO3) 2. What is the Ag+ concentration when BaCrO4 just starts to precipitate? The Ksp for Ag2CrO4 and BaCrO4 are 1.1 × 10-12 and 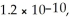 respectively.
respectively.
A) 6.5 × 10-5 mol L-1
B) 1.3 × 10-4 mol L-1
C) 3.2 × 10-4 mol L-1
D) 6.8 × 10-2 mol L-1
Correct Answer:

Verified
Correct Answer:
Verified
Q72: What is the approximate pH at the
Q73: Name the chemical compound that forms stalactites
Q74: What is the pH of a solution
Q75: A 1.50 L buffer solution is 0.250
Q76: What is the pH of a solution
Q78: Calculate the pH of a buffer that
Q79: Calculate the pH of a solution formed
Q80: What is the molar solubility of silver
Q81: Which of the following acids (listed with
Q82: A 100.0 mL sample of 0.18 mol