Multiple Choice
A solution of NaF is added dropwise to a solution that is 0.0188 mol L-1 in 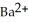 . When the concentration of
. When the concentration of 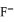 exceeds ________ mol L-1,
exceeds ________ mol L-1, 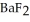 will precipitate. Neglect volume changes. For
will precipitate. Neglect volume changes. For 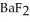 ,
, 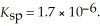
A) 9.0 × 10-5
B) 3.2 × 10-8
C) 4.5 × 10-5
D) 2.4 × 10-3
E) 9.5 × 10-3
Correct Answer:

Verified
Correct Answer:
Verified
Q1: Consider the K<sub>sp</sub> values for two compounds:
Q13: A solution containing CaCl<sub>2</sub> is mixed with
Q14: A 100.0 mL sample of 0.20 mol
Q17: Identify the pH of normal blood.<br>A) 7.0<br>B)
Q19: A sample contains Ba<sub>3</sub>(PO<sub>4</sub>)<sub>2</sub>,<sub> </sub>CdS, AgCl, NH<sub>4</sub>Cl,
Q21: If the pKa of HCOOH is 3.74
Q22: Determine the molar solubility of BaF<sub>2</sub> in
Q23: Which of the following is the correct
Q147: Identify the indicator that can be used
Q173: Match the following.<br>-pH = 7<br>A)equivalence point of