Multiple Choice
The pH of an aqueous solution at 25.0 °C is 10.55. What is the molarity of 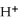 in this solution?
in this solution?
A) 2.8 × 10-11 mol L-1
B) 3.5 × 1010 mol L-1
C) 3.5 × 10-4 mol L-1
D) 3.5 mol L-1
E) 2.36 mol L-1
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q122: Determine the pOH of a 0.741 mol
Q123: Determine the K<sub>a</sub> of an acid whose
Q124: Which of the following is a Bronsted-Lowry
Q125: Which of the following is a Bronsted-Lowry
Q126: Which of the following is a strong
Q128: Calculate the pH of a 0.800 mol
Q129: Calculate the pOH of a solution that
Q130: Which of the following is a Lewis
Q131: Determine the pH of a 0.18 mol
Q132: Which of the following bases is the