Multiple Choice
Determine the ammonia concentration of an aqueous solution that has a pH of 11. 00. The equation for the dissociation of NH3 (Kb = 1.8 × 10-5) is below: 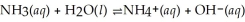
A) 3.0 mol L-1
B) 0. 056 mol L-1
C) 1.8 × 10-2 mol L-1
D) 1.0 × 10-3 mol L-1
Correct Answer:

Verified
Correct Answer:
Verified
Q13: Which of the following bases is the
Q96: What is the conjugate acid of HCO<sub>3</sub>⁻
Q98: Which one of the following salts,when dissolved
Q105: The stronger the acid, _.<br>A) the stronger
Q107: Which one of the following salts, when
Q108: Which of the following is the correct
Q109: Which of the following is the correct
Q113: Which of the following is a weak
Q114: Which of the following increases the strength
Q115: Identify the strongest acid.<br>A) H<sub>2</sub>O<br>B) H<sub>2</sub>S<br>C) H<sub>2</sub>Se<br>D)