Multiple Choice
Express the equilibrium constant for the following reaction: 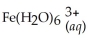 + 6CN-(aq) ⇌
+ 6CN-(aq) ⇌ 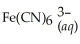 + 6H2O(l)
+ 6H2O(l)
A) Kc = 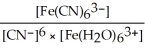
B) Kc = 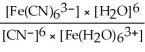
C) Kc = 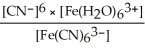
D) Kc = 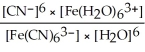
E) Kc = 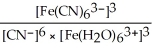
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q3: Give the direction of the reaction if
Q14: Consider the following reaction and its equilibrium
Q15: Determine the value of K<sub>c</sub> for the
Q16: Determine the value of K<sub>c</sub> for the
Q17: For the following reaction, what is △n
Q19: Phosphorus pentachloride decomposes to phosphorus trichloride at
Q21: Determine the value of K<sub>c</sub> for the
Q23: Consider the following reaction at equilibrium. What
Q70: At a certain temperature,nitogen and hydrogen react
Q96: Match the following.<br>-Q < K<br>A)reverse reaction is