Multiple Choice
The Henry's law constant for helium gas in water at 30 °C is 3.70 × 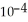 mol L-1 bar-1. When the partial pressure of helium above a sample of water is 0.450 bar, the concentration of helium in the water is ________ mol L-1.
mol L-1 bar-1. When the partial pressure of helium above a sample of water is 0.450 bar, the concentration of helium in the water is ________ mol L-1.
A) 1.22 × 103
B) 8.22 × 10-4
C) 1.67 × 10-4
D) 0.45
E) 3.70 × 10-4
Correct Answer:

Verified
Correct Answer:
Verified
Q94: A solution is prepared by dissolving 38.6
Q113: Which of the following defines dynamic equilibrium
Q114: A compound is found to have a
Q115: Calculate the volume of solution that has
Q117: A nonelectrolyte compound with a molar mass
Q119: Calculate the solution temperature required to produce
Q121: Match the following.<br>-0.050 mol kg<sup>-1</sup> NaCl<br>A)solution of
Q122: Choose the situation below that would result
Q123: Identify the classification of milk.<br>A) aerosol<br>B) solid
Q123: Determine the partial pressure of oxygen necessary