Multiple Choice
At 20 °C, an aqueous solution that is 24.0% by mass in ammonium chloride has a density of 1.0674 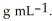 What is the molarity of ammonium chloride in the solution? The formula weight of
What is the molarity of ammonium chloride in the solution? The formula weight of 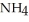 Cl is
Cl is 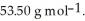
A) 0.209 mol L-1
B) 0.0445 mol L-1
C) 22.5 mol L-1
D) 0.479 mol L-1
E) 4.79 mol L-1
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q27: Give the reason that antifreeze is added
Q73: An unknown nonelectrolyte and nonvolatile compound is
Q75: A 125.0 mL sample of an aqueous
Q76: A mixture of chloroform and acetone has
Q77: A solution is prepared by dissolving 98.6
Q79: A small amount, 6.78 g, of pentane
Q82: A solution containing more solute than the
Q96: What is the molality of a glucose
Q97: Fluids used for an intravenous transfusion must
Q130: Which of the following solutions will have