Multiple Choice
Calculate the freezing point of a solution of 40.0 g salicylaldehyde, C7H6O2, dissolved in 800. g of benzene, C6H6. 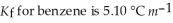 and the freezing point is 5.50 °C for benzene.
and the freezing point is 5.50 °C for benzene.
A) -2.09 °C
B) 2.09 °C
C) 3.41 °C
D) 7.59 °C
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q4: Which of the following statements is TRUE?<br>A)
Q28: What is the mole fraction of oxygen
Q60: A solution of LiCl in water is
Q102: An aqueous solution has a normal boiling
Q105: A solution is prepared by dissolving 16.4
Q106: A solution is 0.0433 mol kg<sup>-1</sup> LiF.
Q107: How many moles of KF are contained
Q108: Commercial grade HCl solutions are typically 39.0%
Q109: Determine the vapour pressure of a solution
Q125: A solution is prepared by dissolving 76.3