Multiple Choice
A solution is prepared by dissolving 21.0 g of glycerin ( 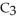
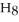
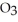 ) in 201 g of ethanol
) in 201 g of ethanol 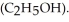 The freezing point of the solution is ________°C. The freezing point of pure ethanol is
The freezing point of the solution is ________°C. The freezing point of pure ethanol is 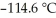 . The molal freezing point depression constant (
. The molal freezing point depression constant ( 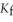 ) for ethanol is
) for ethanol is 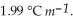 The molar masses of glycerin and of ethanol are 92.1 g mol-1 and 46.1 g mol-1, respectively.
The molar masses of glycerin and of ethanol are 92.1 g mol-1 and 46.1 g mol-1, respectively.
A) -112.8
B) -116.4
C) 2.26
D) -112.3
E) -116.9
Correct Answer:

Verified
Correct Answer:
Verified
Q45: Pentane (C<sub>5</sub>H<sub>12</sub>) is mixed with 75.8 g
Q46: A solid can be purified through which
Q47: Match the following.<br>-0.020 mol kg<sup>-1</sup> NH<sub>4</sub>Cl<br>A)solution of
Q49: Determine the solubility of CO<sub>2</sub> in soda
Q51: Calculate the mass of oxygen (in mg)
Q52: Choose the aqueous solution that has the
Q54: Calculate the vapour pressure of pure water
Q55: Determine the freezing point depression of a
Q80: What mass of CuCl<sub>2</sub> is contained in
Q106: A solution is 2.25% by weight NaHCO<sub>3</sub>.How