Multiple Choice
Consider the phase diagram shown. Choose the statement below that is TRUE. 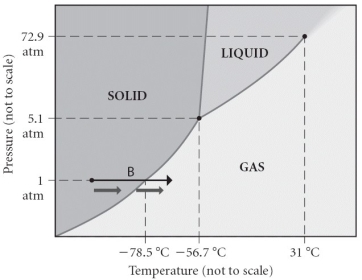
A) The triple point of this substance occurs at a temperature of 31 °C.
B) At 10 bar of pressure, there is no temperature where the liquid phase of this substance would exist.
C) The solid phase of this substance is higher in density than the liquid phase.
D) The line separating the solid and liquid phases represents the ΔvapH.
Correct Answer:

Verified
Correct Answer:
Verified
Q57: Place the following compounds in order of
Q91: Which of the following substances should have
Q106: What is the packing efficiency in simple
Q108: Choose the substance with the lowest vapour
Q110: Which one of the following has a
Q112: Identify the characteristics of a liquid.<br>A) indefinite
Q113: Determine Δ<sub>vap</sub>H for a compound that has
Q114: Why does the temperature of a substance
Q115: Which of the following forms a molecular
Q115: Which of the following statements is FALSE?<br>A)