Multiple Choice
Consider the phase diagram below. If the dashed line at 1 atm of pressure is followed from 100 to 500 °C, what phase changes will occur (in order of increasing temperature) ? 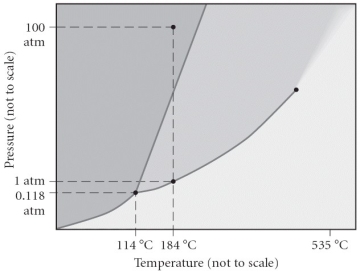
A) condensation followed by vaporization
B) sublimation followed by deposition
C) vaporization followed by deposition
D) fusion followed by vaporization
E) No phase change will occur under the conditions specified.
Correct Answer:

Verified
Correct Answer:
Verified
Q4: Define volatile.
Q6: Place the following compounds in order of
Q60: Ethyl chloride, C<sub>2</sub>H<sub>5</sub>Cl, is used as a
Q61: In liquid ethanol, CH<sub>3</sub>CH<sub>2</sub>OH<sub> </sub><br>Which intermolecular forces
Q62: What is the packing efficiency in body-centred
Q63: Which of the following substances should have
Q66: Choose the pair of substances that are
Q67: Choose the molecule or compound that exhibits
Q69: What is the strongest type of intermolecular
Q70: Why is water an extraordinary substance?<br>A) Water