Multiple Choice
Calculate the total quantity of heat required to convert 25.0 g of liquid CCl4(l) at 35.0 °C to gaseous CCl4 at 76.8 °C (the normal boiling point for CCl4) . The specific heat of CCl4(l) is 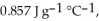 its heat of fusion is
its heat of fusion is 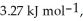 and its heat of vaporization is
and its heat of vaporization is 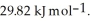
A) 0.896 kJ
B) 1.43 kJ
C) 5.74 kJ
D) 6.28 kJ
Correct Answer:

Verified
Correct Answer:
Verified
Q14: What type of intermolecular force causes the
Q16: Give the coordination number for a body-centred
Q17: Based on the figure above, the boiling
Q20: How much energy is required to vaporize
Q21: Which pairs of substances would form homogeneous
Q22: Identify the compound with the highest boiling
Q23: Determine the vapour pressure (in mbar) of
Q24: Which pairs of substances would form homogeneous
Q43: Choose the pair of substances that are
Q61: Which type of bonding does Sr form