Short Answer
From the phase diagram of benzene given below, predict the temperature at triple point. 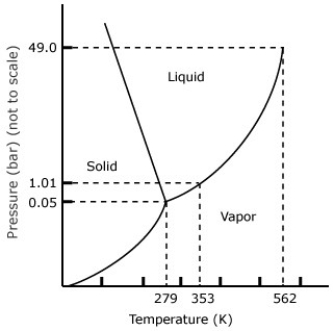
Correct Answer:

Verified
Correct Answer:
Verified
Q6: Which of the following is considered an
Q17: Identify the type of solid for AgCl.<br>A)
Q40: Match the following.<br>-CH<sub>2</sub>F<sub>2 </sub><br>A)dipole-dipole forces<br>B)ion-dipole forces<br>C)H<sub>2</sub> +
Q42: Choose the substance with the lowest viscosity
Q47: How much energy is required to vaporize
Q48: Ethanol ( <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB7901/.jpg" alt="Ethanol (
Q49: Match the following.<br>-LiI + H<sub>2</sub>O<br>A)dipole-dipole forces<br>B)ion-dipole forces<br>C)H<sub>2</sub>
Q50: Identify the compound that does not have
Q104: Place the following substances in order of
Q114: Place the following substances in order of