Multiple Choice
Consider the molecule below. Determine the molecular geometry at each of the three labelled atoms. 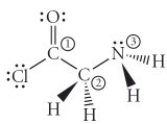
A) 1 = trigonal planar, 2 = tetrahedral, 3 = trigonal pyramidal
B) 1 = tetrahedral, 2 = tetrahedral, 3 = tetrahedral
C) 1 = trigonal planar, 2 = tetrahedral, 3 = tetrahedral
D) 1 = tetrahedral, 2 = tetrahedral, 3 = trigonal planar
E) 1 = trigonal planar, 2 = trigonal pyramidal, 3 = trigonal pyramidal
Correct Answer:

Verified
Correct Answer:
Verified
Q1: What is the O-B-O bond angle in
Q169: Identify the number of electron groups around
Q170: Give the approximate bond angle for a
Q172: Match the following.<br>-XeCl<sub>4</sub><br>A)sp hybridized central atom<br>B)octahedral electron
Q173: Draw the Lewis structure for BrF<sub>5</sub>. What
Q175: Identify the number of electron groups around
Q176: Using the VSEPR model, the electron-domain geometry
Q177: In molecular orbital theory the acronym LUMO
Q178: Determine the molecular geometry for the molecule
Q179: Determine the electron geometry (eg) and molecular