Multiple Choice
Consider the molecule below. Determine the hybridization at each of the two labelled carbons. 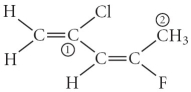
A) C1 = sp3, C2 = sp3d
B) C1 = sp, C2 = sp2
C) C1 = sp2, C2 = sp3d
D) C1 = sp3d, C2 = sp3d2
E) C1 = sp2, C2 = sp3
Correct Answer:

Verified
Correct Answer:
Verified
Q30: The orbital hybridization on the carbon atoms
Q119: Match the following.<br>-SF<sub>4 </sub><br>A)sp hybridized central atom<br>B)octahedral
Q120: List the number of sigma bonds and
Q121: Give the approximate bond angle for a
Q122: Choose the compound below that contains at
Q123: Give the electron geometry (eg), molecular geometry
Q125: A molecule or ion with three electrons
Q126: Draw the Lewis structure for SF<sub>6</sub>. What
Q129: A molecule or ion with four electrons
Q151: Determine the electron geometry (eg), molecular geometry