Multiple Choice
Consider the molecule below. Determine the hybridization at each of the three labelled atoms. 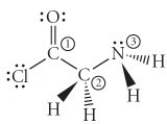
A) 1 = sp2, 2 = sp3, 3 = sp2
B) 1 = sp2, 2 = sp3, 3 = sp3
C) 1 = sp3, 2 = sp3, 3 = sp3
D) 1 = sp3, 2 = sp3, 3 = sp2
E) 1 = sp, 2 = sp2, 3 = sp2
Correct Answer:

Verified
Correct Answer:
Verified
Q9: How many of the following molecules have
Q34: Describe a pi bond.<br>A) side by side
Q72: Use molecular orbital theory to determine whether
Q73: Draw the Lewis structure for BrCl<sub>3</sub>. What
Q75: The hybrid orbital set used by the
Q77: Draw the Lewis structure for H<sub>3</sub>O<sup>+</sup>. What
Q79: Determine the electron geometry for the molecule
Q80: Determine the electron geometry (eg), molecular geometry
Q81: Draw the Lewis structure for the molecule
Q98: Determine the electron geometry (eg)and molecular geometry