Multiple Choice
Use the molecular orbital diagram shown to determine which of the following is most stable. 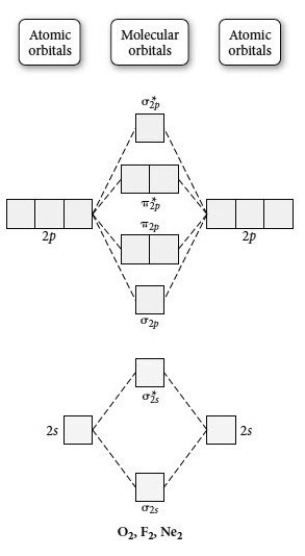
A) F2
B) F22⁺
C) Ne22⁺
D) O22⁺
E) F22⁻
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q17: What are the F-Po-F bond angles in
Q87: Determine the electron geometry (eg) and molecular
Q160: Determine the electron geometry (eg) and molecular
Q161: Draw a molecular orbital diagram and use
Q162: Use the molecular orbital diagram shown to
Q164: Give the approximate bond angle for a
Q167: Determine the electron geometry (eg) and molecular
Q168: Give the approximate bond angle for a
Q169: Identify the number of electron groups around
Q170: Give the approximate bond angle for a