Multiple Choice
Use the molecular orbital diagram shown to determine which of the following is paramagnetic. 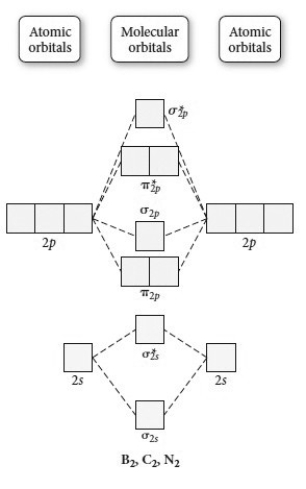
A) B22⁺
B) B22⁻
C) N22⁺
D) C22⁻
E) B2
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q79: Determine the electron geometry for the molecule
Q80: Determine the electron geometry (eg), molecular geometry
Q81: Draw the Lewis structure for the molecule
Q82: How many of the following molecules have
Q83: Using the VSEPR model, the molecular geometry
Q85: Draw the Lewis structure for OF<sub>2</sub>. What
Q86: Draw the Lewis structure for BF<sub>3</sub>. What
Q87: Determine the electron geometry (eg) and molecular
Q88: A molecule containing a central atom with
Q89: Match the following.<br>-sp<br>A)sp hybridized central atom<br>B)octahedral electron