Multiple Choice
Use the data given below to construct a Born-Haber cycle to determine the bond energy of O2. 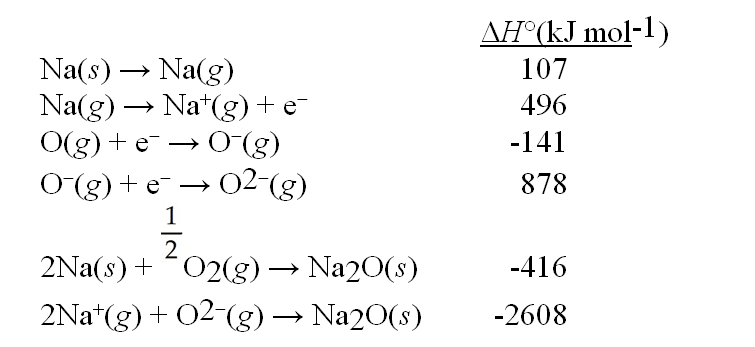
A) 426 kJ mol-1
B) 249 kJ mol-1
C) 852 kJ mol-1
D) 498 kJ mol-1
E) 356 kJ mol-1
Correct Answer:

Verified
Correct Answer:
Verified
Q30: Choose the bond below that is the
Q39: Use Lewis theory to determine the chemical
Q87: Draw the Lewis structure for CO<sub>3</sub><sup>2-</sup>, including
Q88: Choose the best Lewis structure for ICl<sub>5</sub>.<br>A)
Q89: Which of the following represent the Lewis
Q91: Match the following.<br>-C=N<br>A)strongest covalent bond<br>B)longest covalent bond<br>C)weakest
Q93: How many lone pairs of electrons are
Q95: Draw the best Lewis structure for BrO<sub>4</sub>⁻.
Q97: Choose the best Lewis structure for XeI<sub>2</sub>.<br>A)
Q110: Choose the bond below that is the