Multiple Choice
Which of the following processes is endothermic?
A) K⁺(g) + I⁻(g) → KI(s)
B) 2Br(g) → Br2(g)
C) Ca(s) → Ca(g)
D) 2Na(s) + 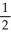 O2(g) → Na2O(s)
O2(g) → Na2O(s)
E) None of the above processes is endothermic.
Correct Answer:

Verified
Correct Answer:
Verified
Q6: Of the following elements,which has the lowest
Q50: Of the following elements,which has the highest
Q66: How many of the following elements can
Q66: Give the number of valence electrons for
Q67: Explain why the lattice energy of MgS
Q68: Choose the best Lewis structure for CH<sub>2</sub>Cl<sub>2</sub>.<br>A)
Q69: Which of the following processes is endothermic?<br>A)
Q70: Choose the best Lewis structure for BeF<sub>2</sub>.<br>A)
Q72: A double covalent bond contains _ of
Q74: Identify the compound with covalent bonding.<br>A) NaF<br>B)