Multiple Choice
Identify the set of four quantum numbers that could represent the electron lost to form the K ION from the K atom.
A) n = 3, l = 1, ml = 1, ms = - 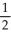
B) n = 4, l = 1, ml = 1, ms = + 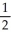
C) n = 4, l = 4, ml = 0, ms = - 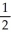
D) n = 4, l = 0, ml = 0, ms = + 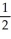
E) n = 3, l = 0, ml = 1, ms = + 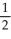
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q35: When waves of equal amplitude from two
Q78: The de Broglie wavelength of an electron
Q79: Calculate the velocity of a pickup truck
Q80: Give the number of valence electrons for
Q81: What are the possible values of n
Q84: Give the value of l for a
Q86: Identify the ground-state electron configuration for the
Q86: Which of the following quantum numbers describes
Q87: How many photons are contained in a
Q96: What is the photoelectric effect?