Multiple Choice
Identify the set of four quantum numbers that could represent the last electron added (using the aufbau principle) to form the Cl atom.
A) n = 3, l = 1, ml = 1, ms = + 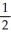
B) n = 3, l = 0, ml = 1, ms = - 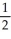
C) n = 3, l = 2, ml =1 , ms = + 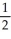
D) n = 2, l = 1, ml = 1, ms = - 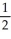
E) n = 3, l =2 , ml = 1, ms = - 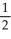
Correct Answer:

Verified
Correct Answer:
Verified
Q15: For n = 3,what are the possible
Q25: Describe the shape of an s orbital.<br>A)
Q27: Calculate the orbital for the hydrogen atom
Q28: How are 3p orbitals different from the
Q29: Identify the set of four quantum numbers
Q32: Identify the ground-state electron configuration for TE<sup>2-</sup>.<br>A)
Q33: Describe how a neon light works.
Q35: Calculate the energy of the red light
Q47: How many subshells are there in the
Q69: What are the possible orbitals for n