Multiple Choice
Identify the set of four quantum numbers that could represent the last electron added (using the aufbau principle) to the Sr atom.
A) n = 5, l = 0, ml = 0, ms = - 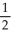
B) n = 4, l = 1, ml = 1, ms = - 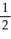
C) n = 5, l = 1, ml = 0, ms = + 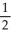
D) n = 4, l = 1, ml = -1, ms = + 
E) n = 5, l = 1, ml =1 , ms = - 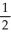
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q18: Calculate the wavelength (in nm)of the red
Q19: Calculate the wavelength of light associated with
Q23: Choose the ground state electron configuration for
Q35: Calculate the energy of the red light
Q37: Which of the following visible colours of
Q39: Which of the following occurs as the
Q41: No two electrons can have the same
Q45: An electron ends in orbital n =
Q107: How many orbitals are contained in the
Q114: Define diffraction.