Multiple Choice
Electromagnetic radiation with a wavelength of 471 nm appears as blue light to the human eye. The energy of one photon of this light is 4.22 × 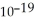 J. Thus, a laser that emits
J. Thus, a laser that emits 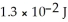 of energy in a pulse of light at this wavelength produces ________ photons in each pulse.
of energy in a pulse of light at this wavelength produces ________ photons in each pulse.
A) 1.5 × 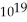
B) 1.2 × 10-23
C) 3.2 × 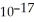
D) 3.1 × 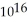
E) 6.5 × 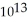
Correct Answer:

Verified
Correct Answer:
Verified
Q59: Choose the paramagnetic species from below.<br>A)Ca<br>B)O<sup>2</sup>⁻<br>C)Cd<sup>2</sup>⁺<br>D)Zn<br>E)Nb<sup>3</sup>⁺
Q75: For a hydrogen atom,which electronic transition would
Q86: Which of the following transitions represent the
Q99: Give the electron configuration for O.<br>A) 1s<sup>2</sup>2s<sup>2</sup>2p<sup>4</sup><br>B)
Q100: Calculate the de Broglie wavelength of a
Q101: Which of the following statements is TRUE?<br>A)
Q103: Place the following types of electromagnetic radiation
Q106: Calculate the energy of the violet light
Q107: Give the number of core electrons for
Q112: What are the possible values of l