Multiple Choice
The complete electron configuration of chromium, element 24, is ________.
A) 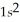
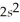
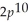
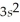 3p8
3p8
B) 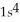
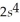
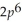
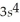 3p6
3p6
C) 1s42s42p103s23p4
D) 1s22s22p63s23p63d54s1
E) 1s42s42p83s43p4
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q59: Choose the paramagnetic species from below.<br>A)Ca<br>B)O<sup>2</sup>⁻<br>C)Cd<sup>2</sup>⁺<br>D)Zn<br>E)Nb<sup>3</sup>⁺
Q82: Which of the following transitions (in a
Q91: Give all possible values of l for
Q92: An electron ends in orbital n =
Q99: Give the electron configuration for O.<br>A) 1s<sup>2</sup>2s<sup>2</sup>2p<sup>4</sup><br>B)
Q100: Calculate the de Broglie wavelength of a
Q104: The vertical height of a wave is
Q112: What are the possible values of l
Q132: Define constructive interference.
Q133: Give the number of valence electrons for