Multiple Choice
The specific heat capacity of liquid water is 4.184 J 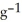
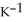 . How many joules of heat are needed to raise the temperature of 7.00 g of water from 33.0 °C to 76.0 °C?
. How many joules of heat are needed to raise the temperature of 7.00 g of water from 33.0 °C to 76.0 °C?
A) 3.19 × 103 J
B) 25.7 J
C) 1.39 × 10-2 J
D) 71.9 J
E) 1.26 × 103 J
Correct Answer:

Verified
Correct Answer:
Verified
Q13: Match the following.<br>--ΔU<br>A)energy flows out of the
Q14: Match the following.<br>-energy associated with the motion
Q15: Match the following.<br>--w<br>A)energy flows out of the
Q16: Sodium metal reacts with water to produce
Q17: A piece of uranium with a mass
Q19: A balloon is inflated from 0.0100 L
Q20: Use the information provided to determine <img
Q21: A 35.6 g sample of ethanol (C<sub>2</sub>H<sub>5</sub>OH)
Q22: Which of the following is not a
Q23: Calculate the internal energy change, Δ<sub>r</sub>U, for