Multiple Choice
If 0.3781 g of a gas with a molecular weight of 18.015 g 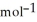 is in a ridged 5.00 L container at 384 K, calculate the final pressure. Assume ideal behaviour.
is in a ridged 5.00 L container at 384 K, calculate the final pressure. Assume ideal behaviour.
A) 0.682 bar
B) 0.109 bar
C) 0.0867 bar
D) 0.132 bar
E) 0.0963 bar
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q90: A syringe contains 0.65 moles of He
Q112: Determine the mass of water formed when
Q113: Identify the gas that is the lowest
Q114: Name the major gas in dry air.
Q116: What is the Celsius temperature of 100.0
Q117: Which of the following would have a
Q118: The mole fraction of argon in dry
Q119: A 0. 286 g sample of gas
Q120: What mass of NO<sub>2</sub> is contained in
Q155: Which of the following samples will have