Multiple Choice
Using the graph below, determine the gas that has the lowest density at STP. 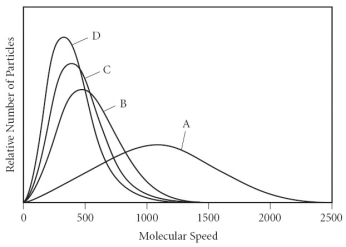
A) A
B) B
C) C
D) D
E) All of the gases have the same density at STP.
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q14: Which of the following statements is TRUE?<br>A)
Q15: What volume will 4.91 × 10<sup>22</sup><sup> </sup>atoms
Q16: The density of a gas is 1.146
Q18: What volume (in mL) will a sample
Q18: If the pressure in a gas container
Q20: An unknown gas has a root mean
Q22: Calculate the temperature if 0.0139 moles of
Q23: Convert 1.25 atm to bar.<br>A) 1.23 bar<br>B)
Q24: Determine the density of O<sub>3</sub> gas at
Q109: A gas mixture consists of N<sub>2</sub>,O<sub>2</sub>,and Ne,where