Multiple Choice
A 0.465 g sample of an unknown compound occupies 245 mL at 298 K and 1.24 bar. What is the molar mass of the unknown compound?
A) 26.3 g 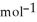
B) 33.9 g 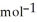
C) 12.2 g 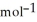
D) 37.9 g 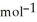
E) 81.8 g 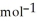
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q19: Rank the following in order of decreasing
Q27: A sample of N<sub>2</sub> effuses in 255
Q119: A 0. 286 g sample of gas
Q120: What mass of NO<sub>2</sub> is contained in
Q121: Convert 123.80 kPa to bar.<br>A) 1.222 bar<br>B)
Q125: Which of the following is FALSE?<br>A) Kinetic
Q126: How many moles of CO are contained
Q127: The volume of a gas is inversely
Q128: A large balloon is initially filled to
Q129: Why doesn't Dalton's law of partial pressures