Multiple Choice
When 14.0 g of zinc metal reacts with excess HCl, how many litres of H2 gas are produced at STP? 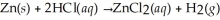
A) 0.208 L
B) 0.416 L
C) 4.86 L
D) 9.60 L
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q55: Which of the following will cause the
Q112: The volume of 350.mL of gas at
Q143: How many molecules of N<sub>2</sub> are in
Q144: What is the lowest layer of the
Q145: How many moles of molecular oxygen are
Q149: Match the following.<br>-Charles's law<br>A)PV = nRT<br>B)V<sub>1</sub>/T<sub>1</sub> =
Q150: How many litres of oxygen are needed
Q151: Calculate the temperature, in K, of 2.20
Q152: Convert 4744.1 mmHg to bar.<br>A) 7.549 bar<br>B)
Q153: Which of the following statements is FALSE?<br>A)