Multiple Choice
Which of the gases in the graph below has the smallest molar mass? 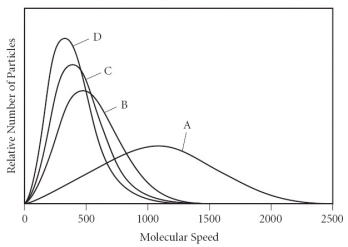
A) A
B) B
C) C
D) D
E) There is not enough information to determine the answer.
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q7: Why does the rate of effusion increase
Q36: What is the definition of diffusion?<br>A) gas
Q38: What is the volume of 9.783 ×
Q39: A syringe initially holds a sample of
Q40: How many grams of F<sub>2</sub> gas are
Q42: Which of the following gases has the
Q43: A syringe contains 589 mL of CO
Q44: Which of the gases in the graph
Q46: Determine the volume of O<sub>2</sub> (at STP)
Q84: Why does hot air rise?