Multiple Choice
Three identical flasks contain three different gases at standard temperature and pressure. Flask A contains 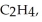 flask B contains O3, and flask C contains F2. Which flask contains the largest number of molecules?
flask B contains O3, and flask C contains F2. Which flask contains the largest number of molecules?
A) flask A
B) flask B
C) flask C
D) All contain the same number of molecules.
Correct Answer:

Verified
Correct Answer:
Verified
Q31: Which of the following samples will have
Q151: Calculate the temperature, in K, of 2.20
Q152: Convert 4744.1 mmHg to bar.<br>A) 7.549 bar<br>B)
Q153: Which of the following statements is FALSE?<br>A)
Q154: Which of the following samples will have
Q157: Match the following.<br>-manometer<br>A)PV = nRT<br>B)V<sub>1</sub>/T<sub>1</sub> = V<sub>2</sub>/T<sub>2</sub><br>C)V<sub>1</sub>/n<sub>1</sub>
Q158: The volume of a gas is proportional
Q159: What is the temperature if krypton has
Q161: The following reaction is used to generate
Q162: Explain what the term "mean free path"