Multiple Choice
A fixed amount of gas at 25.0 °C occupies a volume of 20.0 L when the pressure is 0.8386 bar. Use Charles's law to calculate the volume (L) the gas will occupy when the temperature is increased to 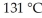 while maintaining the pressure at 0.8386 bar.
while maintaining the pressure at 0.8386 bar.
A) 14.8 L
B) 105 L
C) 3.82 L
D) 22.4 L
E) 27.1 L
Correct Answer:

Verified
Correct Answer:
Verified
Q38: If a sample of 0.29 moles of
Q44: A container filled with gas is connected
Q54: Identify the gas particle that travels the
Q55: Match the following.<br>-Dalton's law<br>A)PV = nRT<br>B)V<sub>1</sub>/T<sub>1</sub> =
Q57: Which one of the following gas laws
Q61: Convert 29.592 inches Hg to bar.<br>A) 0.9889
Q62: Which one of the gas laws explains
Q63: How many molecules of XeF<sub>6 </sub>are formed
Q76: You have three cylinders containing O<sub>2</sub> gas
Q172: Define effusion.